...and why it's not really wasteful
If you have ever wondered why living cells sometimes seem to waste their resources, you are not alone. For over a century, scientists have been interested in an intriguing phenomenon called “metabolic overflow”. This occurs when cells—whether bacteria, yeast, or even our own human cells—consume nutrients such as sugars but do not use them entirely to meet their energy needs and multiply. Instead, they release some of these resources into their environment in the form of by-products that appear useless and sometimes even toxic.
At first glance, this strategy seems inefficient. Why would a cell, shaped by evolution to maximise its survival and growth, reject valuable fuel in the form of harmful compounds?
In our recent review (Gosselin-Monplaisir et al., Molecular Systems Biology, 2025), we revisit this mystery. We show that metabolic overflow operates according to universal biological principles observed in all areas of life, and is neither an error nor a weakness. Far from being mere toxic waste, these by-products even play essential roles as nutrients, regulators and signalling molecules.
Different names for the same process
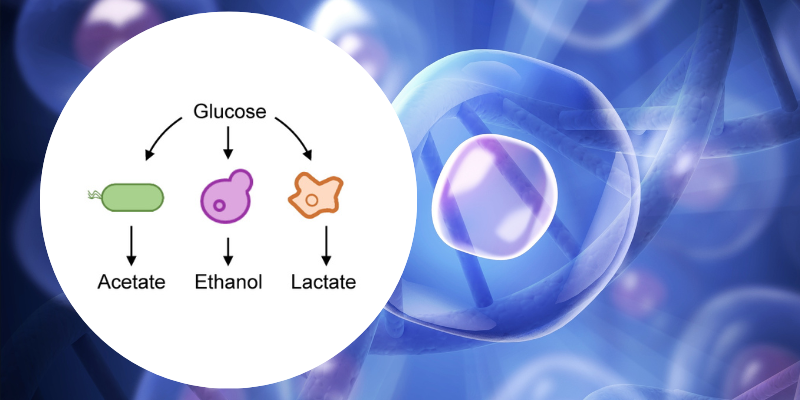
Metabolic overflow has been observed in almost all organisms studied. However, as it has been studied independently in different species, scientists have given it different names:
- In bacteria such as Escherichia coli, we often talk about acetate overflow, where sugar is converted into acetic acid, the same compound found in vinegar.
- In yeast, this is called the Crabtree effect, and the sugar is converted into ethanol — the process used to make beer and wine.
- In animal cells, this is known as the Warburg effect, where sugar is converted into lactate.
For decades, these processes were considered to be largely independent. Our review shows that they are in fact variants of the same fundamental process, functioning in remarkably similar ways throughout the living world.
Metabolic overflow follows universal principles
By analysing, comparing and integrating studies on bacteria, yeasts and animal cells, we have identified principles that are conserved in all organisms:
- Reversibility: metabolic overflow is not a one-way street. The molecules produced (such as lactate, acetate or ethanol) can be reabsorbed and reused by cells. It is therefore a reversible process that allows cells to decouple energy production from sugar utilisation.
- Shared control: metabolic overflow is not a process controlled by a single enzyme or metabolic pathway. Its functioning is determined by the interaction between three universal metabolic systems: glycolysis (which breaks down sugars), the Krebs cycle (which converts sugars into energy), and the overflow pathways themselves.
- Thermodynamics: fundamental physical constraints force cells to excrete some of the nutrients when they use them quickly, at least when there are no by-products. On the other hand, an increase in the concentration of by-products drives the reaction in the opposite direction, allowing cells to use what they previously excreted along with sugar. Thus, by-products can act as ‘waste’ in some contexts and as nutrients in others, depending on the availability of sugars and by-products.
- Adaptation: by-products can make cells more resilient to changes in their environment. Acetate helps bacteria cope with variations in sugar availability, ethanol provides a backup energy source for yeast, and lactate allows animal cells to share energy between organs. Far from being solely harmful, these molecules also confer benefits on the cells that produce them.
These results thus reveal a universal flexible strategy: evolution has optimised cells not only for efficiency, but also for adaptability, sometimes at the cost of short-term “waste” of resources, in order to ensure long-term survival advantages. This perspective lays the foundations for a unified theory of metabolic overflow, which has been lacking in biology until now.
Not just toxic waste: nutrients, regulators and messengers
One of the most important changes in perspective is recognising that by-products of metabolic overflow are not simply toxic waste:
- In bacteria, acetate can regulate metabolism, help maintain nutritional balance, coordinate collective behaviours such as biofilm formation, and even improve growth.
- In yeast, ethanol triggers a global cellular response and supports its energy needs.
- In human health, lactate, long accused of causing muscle fatigue, is now recognised as a signalling molecule and vital nutrient, redistributed between organs. It also plays a key role in cancer metabolism, immune response and brain function.
Thus, far from being mere waste products, these molecules can also act as nutrients, regulators and messengers, enabling cells to communicate and adapt collectively to a constantly changing environment. Only by recognising the diversity of their roles, which are highly context-dependent, can we fully understand the phenomenon of metabolic overflow.
Why it matters
Understanding metabolic overflow is not just a theoretical question, it is also a practical issue.
In biotechnology, where microorganisms are used to produce food, fuel or medicines, the accumulation of these by-products can reduce productivity. By applying the principles identified here, more efficient bioprocesses can be designed to minimise the production of these by-products or recycle them into high value-added compounds.
In healthy individuals, metabolic by-products circulate continuously between our cells, organs and gut microbiota. Disruption of these exchanges is linked to diseases such as cancer, obesity and microbiota disorders. A better understanding of this metabolism could therefore pave the way for new diagnostics and therapies.
Outlook
Metabolic overflow is not a flaw in cellular design but a universal process that organisms, from bacteria to humans, use to survive in an ever-changing world. By continuing our study across different species, we may discover that the production of this ‘waste,’ which we have long sought to eliminate, is in fact a powerful driver of resilience in living organisms, and that it can be harnessed to develop more robust, efficient, and sustainable bioprocesses.
References
Thomas Gosselin-Monplaisir, Brice Enjalbert, Sandrine Uttenweiler-Joseph, Jean-Charles Portais, Stéphanie Heux, and Pierre Millard (2025). Overflow metabolism in bacterial, yeast, and mammalian cells: different names, same game . Mol Syst Biol, DOI : https://doi.org/10.1038/s44320-025-00145-x
Contact
Pierre MILLARD